
How do electrolysers work?
Electrolysers are electrochemical devices that use electricity to split water into hydrogen and oxygen, a process known as electrolysis. When electrolysis is powered with renewable electricity it produces green hydrogen, which is now widely recognised as key to enabling the energy transition.
As with other electrochemical devices such as batteries and fuel cells, electrolysers consist of an anode and a cathode separated by an electrolyte. Electrolysers function in different ways depending on the type of electrolyte material and the ionic conductor. Examples include alkaline, polymer electrolyte membrane (PEM), and solid oxide electrolysers.
As with other electrochemical devices such as batteries and fuel cells, electrolysers consist of an anode and a cathode separated by an electrolyte.
Alkaline electrolysers are the most widely available. The electrolyte is a liquid alkaline solution of sodium or potassium hydroxide and the electrodes are coated metal wires. Applying a current to the solution causes water molecules at the cathode to gain electrons. This splits them into hydrogen gas and negatively charged hydroxide ions. Hydroxide ions move through the electrolyte from the cathode to the anode, where they lose electrons back to the circuit and form oxygen gas and water molecules. This technology has proven to be reliable and low cost.
PEM electrolysers use a very thin polymer membrane as a solid electrolyte. Water reacts at the anode to form oxygen and positively charged hydrogen ions, which then move across the PEM to the cathode. Electrons flow through an external circuit to the cathode, where they combine with the hydrogen ions to form hydrogen gas. The technology processes more power in a smaller space and can adjust its output depending on the electricity available. Coupling PEM with intermittent renewables makes sense where operators are prepared to pay a higher price for dynamic response and a compact footprint.
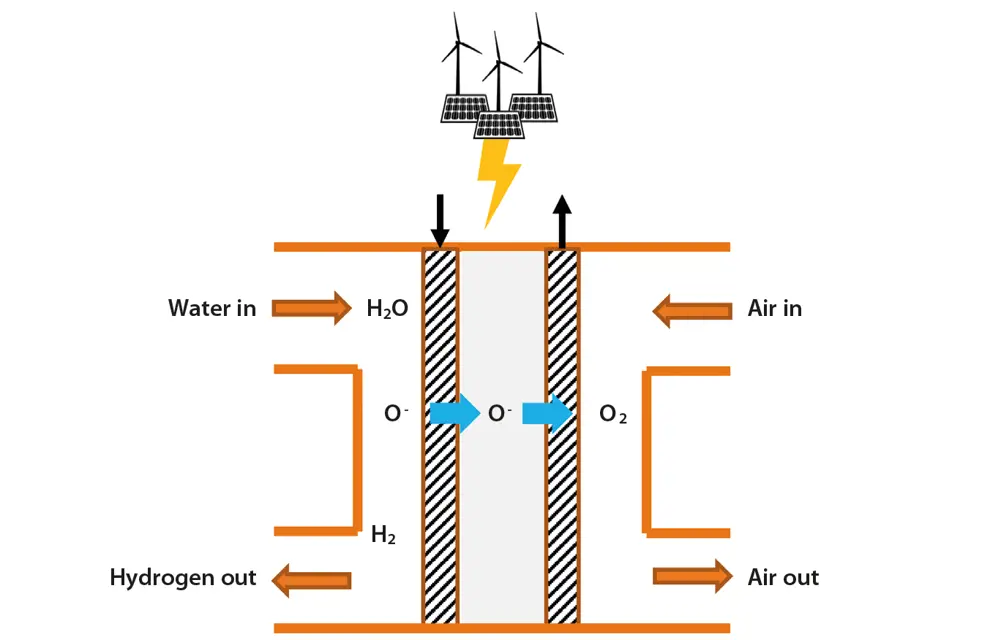
Solid oxide electrolysis (SOEC), such as that developed by Ceres Power, is a high-temperature technology that uses a solid ceramic electrolyte. Steam at the cathode combines with electrons from the external circuit to form hydrogen gas and negatively charged oxygen ions. These ions pass through the solid ceramic membrane, forming oxygen gas at the anode and generating electrons for the external circuit. It is about 25% more efficient than incumbent low temperature technologies and can make use of residual heat from industrial processes. While best-in-class alkaline or PEM technology would require about 50 kWh of electricity to produce a kilogram of hydrogen, SOEC only needs 37 kWh of electricity.
Green hydrogen can be turned back into electricity when demand is high (potentially through a fuel cell, essentially an electrolyser in reverse). It can also be used as a precursor to other sustainable fuels where higher energy density is required, such as in shipping or aviation. Methanol, ammonia and methane can all be synthesised from hydrogen and are being considered as future energy carriers and in zero carbon power generation.
While the debate continues about the full extent of global hydrogen applications, it will be key in decarbonising essential but emissions-intensive industries such as steelmaking, cement and marine transport.
While the debate continues about the full extent of global hydrogen applications, it will be key in decarbonising essential but emissions-intensive industries such as steelmaking, cement and marine transport. Evidence is also growing for a major role in long-term energy storage for renewables. Electrolyser technology can play a significant role in reaching net zero.
Companies around the world are increasing electrolyser production, green hydrogen plants are under construction, and the industry is transitioning from pilot to industrial projects. As with solar, wind and battery technologies, these actions will be key to driving the scale needed to reduce costs and ensure we get electrolysers from being tomorrow’s technology to part of the infrastructure of our society.
Words by Ceres Power
***
This article has been adapted from "How does that work – Electrolysers", which originally appeared in the print edition of Ingenia 96 (September 2023).
Contributors
Ceres Power
Author
Keep up-to-date with Ingenia for free
SubscribeRelated content
Energy

Algae-powered architecture
An apartment block in Hamburg in Germany has been built that uses microalgae placed within its façade to generate heat and biomass. Jan Wurm, an associate director at Arup, was one of the chief designers of the energy system. He talked about the concept, execution and results from the world’s first photobioreactor.

Digital hydraulics for wind energy and beyond
Research that has helped change the technology for harnessing wind energy has many other applications. The digital hydraulics system devised by Artemis Intelligent Power has received many accolades, the latest being the winner of the 2015 MacRobert Award.

New energy pioneers
London-based BBOXX supplies solar-powered battery boxes to customers in developing countries. Their remote monitoring and battery management system was one of the winners of the 2015 Bloomberg New Energy Finance Award.

Energy with connections
When Steve Holliday FREng moved from the oil industry into energy distribution, the sector was seen as staid. In reality, during his years at National Grid, the sector became increasingly important as the need to tackle climate change led to a transformation in the UK’s energy mix.
Other content from Ingenia
Quick read

- Environment & sustainability
- Opinion
A young engineer’s perspective on the good, the bad and the ugly of COP27

- Environment & sustainability
- Issue 95
How do we pay for net zero technologies?
Quick read

- Transport
- Mechanical
- How I got here
Electrifying trains and STEMAZING outreach

- Civil & structural
- Environment & sustainability
- Issue 95